Inflammation classically has been viewed as an immediate high-level and short-term (“acute”) response to tissue injury, infection, or other disease states, which leads to recognizable symptoms such as swelling, redness, and warmth in the affected area. It helps the body heal and defend itself.
However, in the 1990s, researchers began to explore the potential link between inflammation and age-related diseases, such as cardiovascular disease and Alzheimer’s disease. This resulted in inflammaging being coined in a 2000 article[1].
The article proposed that inflammaging was fundamental in the ageing process, suggesting that long-term (“chronic”) low-grade inflammation was a key factor contributing to age-related diseases.
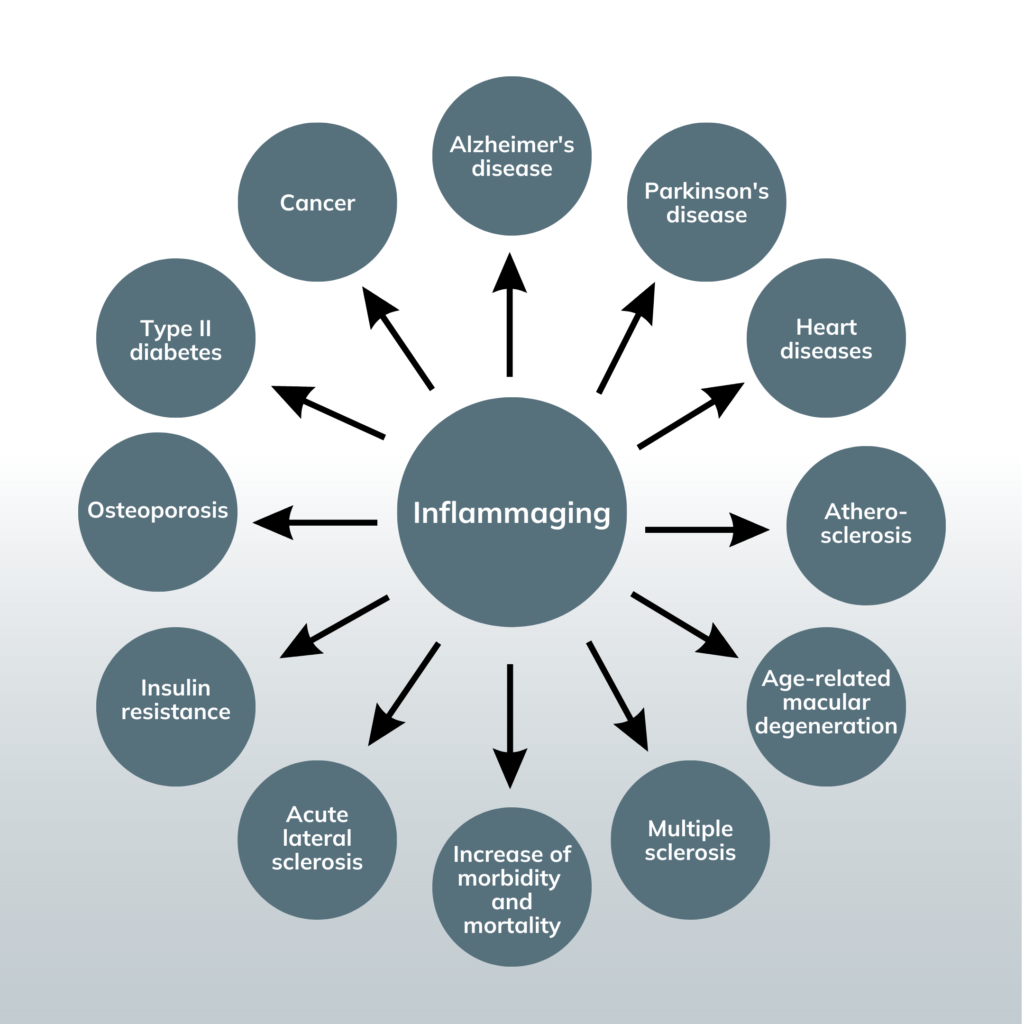
The inflammaging hypothesis has gained traction in recent years as researchers have identified a wide range of age-related diseases associated with chronic low-level inflammation.
The primary theory behind inflammaging is that when cells become damaged or reach the end of their lifespan, the immune system usually clears them from the body. However, as we age, our immune system becomes less efficient at this process, leading to the accumulation of damaged cells and cellular debris.
This accumulation can trigger an immune response, including the activation of pro-inflammatory cytokines and other immune cells, leading to chronic inflammation.
Additionally, the innate immune system, which plays a critical role in responding to infections, injuries, and toxins, can contribute to chronic inflammation as we age. This is because the innate immune system can become increasingly active and remain in a persistent state of alertness, even in the absence of immediate threats. This hypervigilant immune response may also contribute to chronic inflammation.
Below are factors now known to contribute to inflammaging:
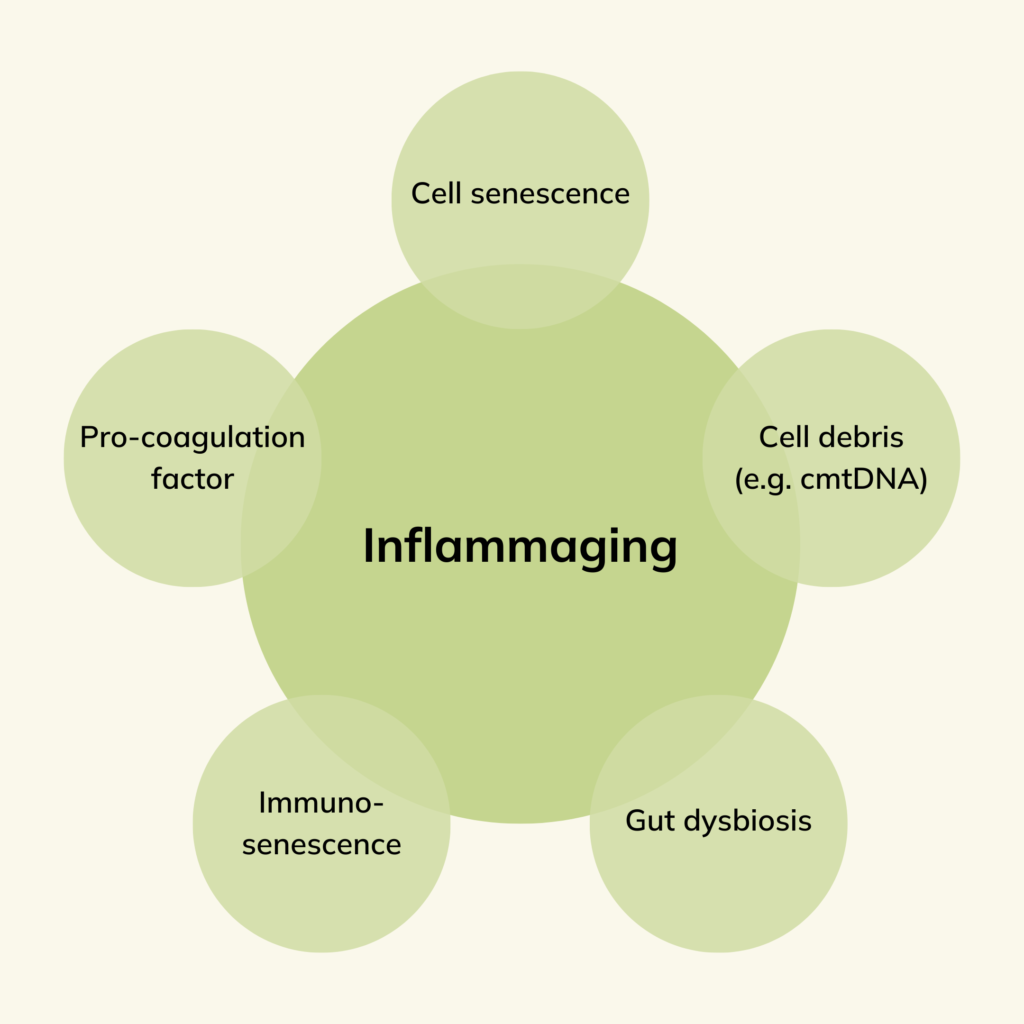
- Accumulation of senescent cells
- Increased production of pro-inflammatory cytokines and chemokines
- Changes in the gut microbiome, leading to gut dysbiosis and increased gut permeability
- Activation of the inflammasome, a protein complex that plays a role in inflammation
- Oxidative stress and DNA damage
- Dysregulation of the complement system, a group of proteins that play a role in immune defence and inflammation
- Activation of the innate immune system
- Epigenetic changes, including DNA methylation and histone modification, that can alter gene expression and contribute to inflammation
- Accumulation of cellular debris, including mitochondrial DNA
Gut dysbiosis and increased gut permeability are more recent additions. The first relates to changes in the composition and diversity of microorganisms in the gut. This imbalance in favour of harmful bacteria can activate the immune system and trigger an inflammatory response, contributing to chronic inflammation. The second relates to leaky gut syndrome, where the gut lining becomes more permeable, allowing harmful substances to leak into the bloodstream, triggering an inflammatory response.

Environmental stressors such as pollution have been shown to increase chronic inflammation, as have lifestyle factors such as smoking, lack of exercise, alcohol consumption, and poor diet.
Chronic inflammation can negatively affect your body, such as increased oxidative stress, impaired cellular function, and tissue damage. These outcomes can further contribute to chronic inflammation, leading to a vicious cycle of damage and inflammation. Chronic inflammation is associated with the development of at least 7 of the top 10 leading causes of death.
To assess chronic inflammation, specific biomarkers such as C-reactive protein (CRP), interleukin-6 (IL-6), tumour necrosis factor-alpha (TNF-alpha), and fibrinogen can be measured. Research has shown that levels of these biomarkers have an inverse correlation with both your health span and lifespan.
In other words, consistently high levels of inflammation in any of these biomarkers may indicate a chronic state of inflammation, which can reduce your overall health and longevity.
Dysbiosis
The gut microbiota refers to the community of microorganisms – mostly bacteria – that live within our gastrointestinal tract, primarily in the large intestine. These microorganisms include bacteria, v…
References









