Here’s a fun thought: you’re ageing. Yes, even though you’re in your prime youth, your cells are busy ticking away, marking the passage of time. And truth be old, your ageing process can be accelerated by the lifestyle you’ve been leading for the past few years.
Now, before you let that thought stress you into ageing even faster, let’s pause. What if you could understand the inner workings of the ageing process and use that knowledge to maximise your health span? That’s the time you spend being healthy and active, by the way.
Imagine yourself celebrating your 80th birthday with a skydive instead of a cane? The key here is to understand, at least to some degree, how your body ages. Scientists have established 12 hallmarks of ageing– 12 mechanisms that pave the way for you to grow.
Let’s get you familiarised, so you can tweak them in your favour.
In this article
Free guide to reverse your biological age

- Master the science of rejuvenation.
- Apply proven tips to turn back the clock.
- Transform your health with top longevity specialists.
What does a hallmark of ageing even mean?
3 criteria define a hallmark of ageing:
- its time-dependent manifestation
- the potential for experimental acceleration of ageing
- and most critically, the potential for therapeutic intervention to slow or reverse ageing.
The hallmarks provide us with a framework for exploring the complexity of ageing on molecular, cellular, and systemic levels. They also act as gateways for developing new anti-ageing interventions.
The updated list reflects the increasingly sophisticated tools like ‘omics’ technologies that we now have at our disposal for studying ageing, and the expanding evidence base, now including mammalian models.
The framework for the hallmarks of ageing serve as clinical relevance. Diseases linked to the same hallmark are more likely to co-occur and share genomic features, reinforcing the approach taken [1].
Let’s break down each hallmark, and understand its relation to ageing as well as age-related diseases.
What are the 12 hallmarks of ageing?
Since the groundbreaking publication of the hallmarks of ageing in 2013, the gerontology field has exploded, generating nearly 300,000 studies—matching the output of the entire preceding century.
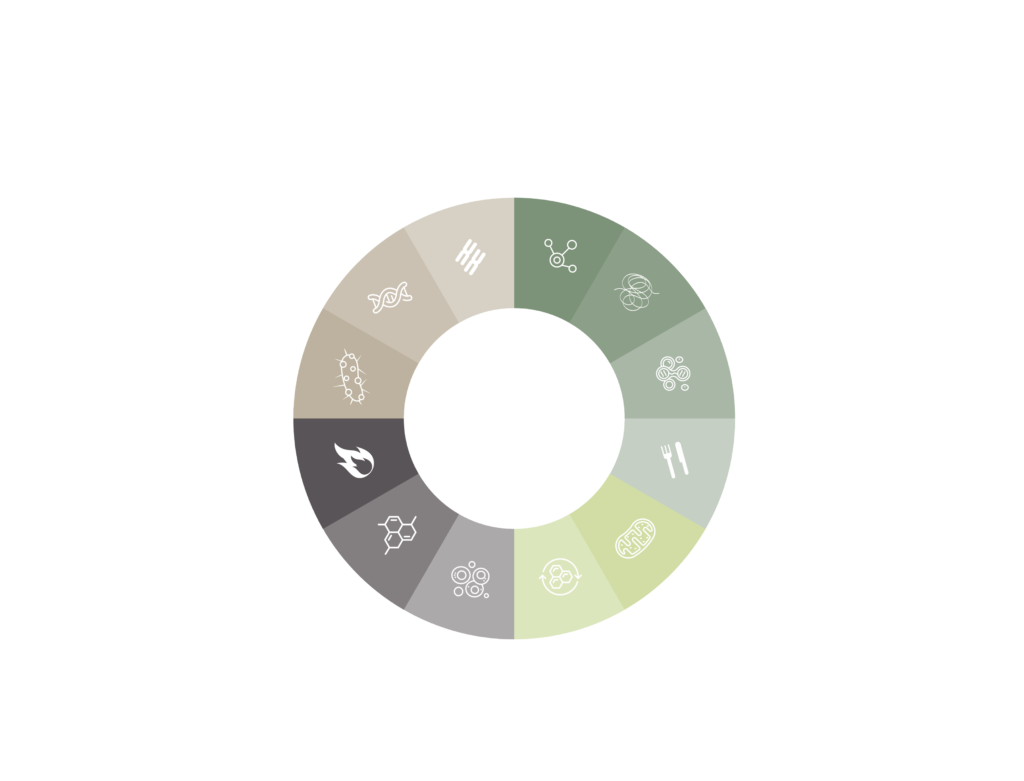
As scientists gain more insights, the need for an updated version of these hallmarks became evident. Originally, 9 core hallmarks were outlined:
- DNA instability
- telomere attrition
- epigenetic alterations
- loss of proteostasis
- deregulated nutrient-sensing
- mitochondrial dysfunction
- cellular senescence
- stem cell exhaustion
- altered intercellular communication
These aren’t isolated processes; they intertwine and influence one another. In updating the hallmarks, 3 more were added to the list:
- disabled macroautophagy
- chronic inflammation
- dysbiosis
1. Genomic instability
Genomic instability essentially means your DNA is not as stable as it should be. It’s not just about the occasional DNA mishap; but includes alterations ranging from point mutations and deletions to chromosomal rearrangements. Our cellular DNA faces internal and external threats, including replication errors and oxidative processes.
As you age, the DNA repair mechanisms, your cellular caretakers, lose their efficiency. This is particularly problematic in stem cells, which are responsible for tissue renewal. Accumulated DNA damage can lead to tissue degeneration and increased susceptibility to diseases.
Findings suggest that animals with lower rates of DNA mutations tend to live longer. Although more studies are required, it is safe to say that if we can find a way to keep our DNA more stable throughout our lives, we may find ourselves on a path to extended health span and maybe even lifespan.
One molecule to watch is Sirtuin-6 (SIRT6), which has been shown to enhance DNA repair and extend lifespan in mice. Lastly, it’s not just the DNA in the nucleus of the cell we have to worry about; mitochondrial DNA is also at risk. Here, too, better repair mechanisms might offer a way to slow down ageing.
2. Telomere shortening
At the end of each chromosome, you’ll find a region called a telomere. These telomeres are like the aglets on shoelaces—they stop the DNA from fraying.
But as cells divide, these telomeres shorten, leading to genomic instability, pushing cells towards either self-destruction (apoptosis) or a non-dividing state (senescence). And as you can guess, neither is good for longevity. Telomeres are affected by age, genetics, lifestyle, and even social factors.
Now, nature did provide a saviour: telomerase. This is an enzyme that can elongate the telomeres, thereby delaying these deleterious effects.
The issue is that most of our cells don’t express enough of it, which allows telomeres to shorten over time. So it’s like having a repair tool, but keeping it locked in the shed.
Telomere attrition has a silver lining in that it can limit the lifespan of malignant cells, making it harder for cancer to develop. So, unlike genomic instability, which is straightforwardly bad, telomere attrition has a dual role, with scientists hoping to harness its benefits.
Research has shown that telomere length can predict lifespan across species. There are also proteins called shelterins that help maintain telomere integrity. Any deficiencies in these proteins can lead to premature ageing, regardless of telomere length.
Now, onto the exciting part: interventions. Studies in mice show that activating telomerase can extend lifespan and improve health. It’s like giving that locked-away tool to the cells that need it.
In humans, short telomeres have been linked to diseases like pulmonary fibrosis and kidney fibrosis, so activating telomerase could potentially delay these age-related diseases.
3. Epigenetic alterations
If you think of the genome as a complex blueprint for life, epigenetic modifications are akin to the annotations and highlighter marks on that blueprint.
These tiny changes, often reversible, can profoundly impact how genes are read, or expressed, by cells. In ageing, it’s like the annotations start to become confusing, leading to a more chaotic and less efficient cellular operation.
Learn how epigenetics affect your health.
One of the primary epigenetic changes observed in ageing is DNA methylation—the addition of methyl groups to the DNA molecule.
Imagine DNA methylation as the dimmer switch of the genomic household. Too much or too little light can cause problems.
As we age, some areas of the genome become hypermethylated, essentially turning off crucial genes. On the flip side, some become hypomethylated, leading to the activation of genes that should be dormant. The balance is disrupted, leading to an increased risk of diseases like cancer and a general state of cellular decline.
Histones are another key player. These are proteins that help to package DNA neatly. But overtime, the modifications on histones can change (acetylation and methylation of histones), making the genome either too loose or too tight.
This can lead to inappropriate expression of genes. Some proteins like the sirtuins try to restore balance, but their effectiveness may decline with age too, tipping the scale further into chaos.
Then there are the non-coding RNAs, often overlooked but increasingly recognised as significant players in ageing. These RNA molecules can either obstruct or enhance the expression of specific genes. As we age, the levels and types of non-coding RNAs can change, contributing to age-related diseases and cellular decline.
All these epigenetic elements culminate in changes in gene expression, which manifest as ageing. The process becomes more chaotic over time, affecting critical biological pathways like inflammation and mitochondrial function.

What’s particularly exciting is that many epigenetic changes can be reversed.
This opens up the possibility of therapeutically targeting epigenetic changes to either slow down or perhaps even reverse aspects of ageing.
4. Loss of proteostasis
Proteostasis, or protein homeostasis, is a fine-tuned system of protein synthesis, folding, and degradation. This cellular mechanism is vital for maintaining the structure and function of proteins in our body.
As we age, this system can become less efficient, resulting in an accumulation of misfolded or damaged proteins. This creates a biochemical mess, with ties to age-related diseases like Alzheimer’s and Parkinson’s.
Misfolded proteins can clog the cellular machinery and disrupt essential cellular functions. The impaired protein translation directly impacts lifespan across various organisms.
Oxidative stress is another culprit. It further distracts cellular chaperones, which are supposed to assist in protein folding, thereby exacerbating the problem.
Over time, the system becomes increasingly inefficient, leading to more accumulation of mostly flawed proteins.
What’s worse is that proteostasis relies heavily on quality control mechanisms like the unfolded protein response (UPR) in the endoplasmic reticulum (ER).
As the effectiveness of UPR declines overtime, another layer of quality control is weakened. Similarly, reductions in proteasome activity, the cellular rubbish disposal for proteins, have been observed in aged tissues.
Chaperone-mediated autophagy (CMA) plays a role in selective degradation of proteins. In a nutshell, it’s a system to degrade and remove the unwanted proteins, but its efficiency also decreases with time, contributing to ageing and diseases like Alzheimer’s.
Strategies like stimulating autophagy to remove protein aggregates or enhancing CMA have shown promise in ameliorating aspects of ageing. Mice showed improved proteostasis and lifespan.
Learn how to promote autophagy with 4 easy, science-backed steps.
5. Disabled macrophagy
When autophagy is disabled or compromised, it’s akin to having a broken garbage disposal system. We start to see a pile-up of dysfunctional cellular machinery, from protein aggregates to used mitochondria.
This littering in the cellular environment impacts everything from the cell’s ability to generate energy via mitochondria to the effective elimination of pathogens. Decreased autophagic activity has been observed with ageing.
Disabled autophagy leads to inflammation spikes, cellular function deteriorates, and susceptibility to diseases increases. It is clear that the absence of a well-functioning autophagy process is a significant issue in the context of ageing and longevity.
It is even linked to an increased cancer incidence. Meaning, autophagy not only maintains cellular health but also surveils and regulates potentially malignant activities.
Stimulating autophagy has shown considerable promise in animal models.

Spermidine supplementation increases lifespan in mice by 25% and reduces cardiac ageing.
Discover 8 Spermidine benefits you should know about.
NAD+ precursors (nicotinamide, nicotinamide mononucleotide, nicotinamide riboside) and urolithin A have shown similar positive impacts, opening avenues for more therapeutic interventions.
What is NAD+ and why is it important?
What is especially noteworthy for the longevity sector is how these interventions may not just slow down the ageing process, but actively reverse aspects of it.
6. Deregulated nutrient-sensing
Nutrient-sensing pathways have intrigued scientists for years for their role in metabolic modulation and ageing.

Did you know that some mechanisms are known to respond to nutrients and stressors alike to regulate cellular activity, including autophagy and metabolism?
These pathways involve hundreds of molecules, forming a network crucial for maintaining cellular function, particularly as we age.
When we are young, the nutrient-sensing network promotes anabolic processes—those that build cellular components. But the tables turn as we age.
The very same mechanisms that once helped us grow and repair now acquire pro-ageing properties. This switch raises the question: could we ‘reprogram’ this network to work in our favour as we age?
Genetic studies in animal models have shown that reduced activity of some molecules can actually extend lifespan. Similar results have been observed in humans.
Supplements like NAD+ and dietary interventions like fasting can also mimic these beneficial genetic ‘quirks’ to improve our own health span.
Both have shown promise in extending lifespan in animal models but also indicate potential benefits for human health. More studies are needed for dietary interventions, since the male and female body are built differently at biological level.
The prospect of targeting nutrient-sensing pathways could revolutionise our approach to combating age-related diseases and extending human lifespan.
7. Mitochondrial dysfunction
Mitochondria have long been celebrated as the powerhouses of the cell. Yet, their role extends far beyond that: they also serve as regulators of inflammation and cell death.
With ageing, mitochondrial function declines due to several factors, ranging from mitochondrial DNA (mtDNA) mutations, destabilisation of respiratory chain complexes, diminished organelle turnover, and altered mitochondrial dynamics.
Such dysfunction triggers the release of molecules that activate various pathways, including inflammasomes and cytosolic DNA sensors.
These pathways not only hinder cellular energy production but also perpetuate inflammation and cell death, adding a multifaceted layer of complexity to the ageing process.
While one might think enhancing mitochondrial function is the only key to a longer, healthier life, an intriguing phenomenon known as “mitohormesis” offers a different perspective.
Mild inhibition of mitochondrial activity—particularly in early developmental stages—induces a hormetic response that can extend lifespan in model organisms like C. elegans and Drosophila.
Clinical trials have reported the positive effects of certain supplementation on pre-frail and elderly subjects.
Plasma levels of mitochondrial microproteins are also gaining attention for their potential in inducing autophagy. These protein levels, which decline with age, are unusually high among centenarians and their offspring.
While animal and cellular models provide valuable insights, the translation of these findings to human health and longevity remains a hard yet exciting challenge.
8. Cellular senescence
Cellular senescence occurs when our cells officially “retire” and stop dividing– also known as ‘’zombie cells’’. This occurs more as you age, and it can happen in any type of cell. Once thought to just be a way to prevent cancer, but it’s now also seen as a cause of ageing and other diseases.
Cellular senescence occurs due to damage to their DNA, telomere shortening, and other types of stress like infection. Originally, it is a useful response to damage—sort of like calling a timeout and bringing in the immune system to clean up. But as we age or in cases of chronic disease, the clean-up process isn’t as efficient.
These cells can cause nearby cells to also become senescent, leading to a build-up of these unhealthy cells. They produce a range of chemicals that can cause inflammation and fibrosis (thickening and scarring of connective tissue). They also play a role in diseases like diabetes, Alzheimer’s, and many more.
Cellular senescence is managed by certain internal proteins, which stop the cell from dividing and change its behaviour. While there’s no one-size-fits-all test to identify senescent cells, you can look for a mix of features like certain proteins, chemicals, and other cell behaviours to make an educated guess.
That’s where “senolytics” come in. These are drugs designed to selectively kill these senescent cells. Some have shown promise in treating diseases and are in various stages of clinical trials.
Learn more about senolytics here.
9. Stem cell exhaustion
Ageing is associated with reduced tissue renewal at steady state, as well as with impaired tissue repair upon injury, with each organ having its own strategy for renewal and repair.
As you get older, your body is less effective at repairing itself, both in terms of general upkeep and healing from injuries. Different organs have their own ways to repair and renew.
- In skeletal muscle, one single-cell type, the satellite cell, takes the lead in both day-to-day renewal and repair.
- In skin, characterized by high renewal and exposure to injury, there are multiple stem cell ‘neighbourhoods,’ especially around hair follicles.
- Other organs like liver, lung, or pancreas exhibit rather low renewal rates under normal conditions.
When you’re injured, different types of cells can temporarily act like stem cells to help repair the damage.

The ability of cells to adapt and change in response to injury might be more relevant to ageing than how flexible our ‘everyday’ stem cells are.
Just like all other cells, stem cells and their “baby cells,” called progenitors, also age. When the pool of these cells is reduced, your body can’t repair itself properly, leading to ageing and making you more susceptible to diseases.
Cellular reprogramming turns adult cells back into a state similar to embryonic stem cells using specific ‘gene switches’ (transcription factors).
Natural tissue repair and artificial reprogramming share features that could lead to new ways to rejuvenate ageing tissues.
Reprogramming can help in extending lifespan of the cell, affecting various markers of ageing such as DNA damage and epigenetic patterns.
In experiments with mice, reprogramming made older tissues repair themselves as efficiently as the tissues in young mice.
10: Chronic inflammation
Ah, inflammation is the best example of a double-edged sword– there’s no doubting that. Inflammation is part of your body’s natural defence mechanism, but too much of it, particularly as you age, could be problematic.
Let’s talk about “inflammaging,” a term coined to describe the chronic low-level inflammation that accompanies ageing. When we talk about ageing, circulating levels of inflammatory markers often come into play.

Elevated inflammatory markers are a strong predictor of all-cause mortality in ageing populations.
A heightened inflammation status contributes to an impaired immune function, potentially rendering the body more susceptible to infections, cancer, and autoimmune diseases. Not a good look for your golden years, to say the least.
Inflammation doesn’t occur in isolation. It’s intricately connected with other hallmarks of ageing, such as genomic instability, dysfunctional autophagy, and poor proteostasis.
For instance, genomic mutations can lead to a condition called clonal hematopoiesis, where a type of blood cell start to multiply unusually, leading to inflammation, and accelerating cardiovascular ageing.
Anti-inflammatory treatments have shown promise in preclinical models, including different manipulations across different organ systems.
11: Dysbiosis
The intricate relationship between gut microbiota and ageing is a subject of increasing interest in scientific research. On one hand, the gut microbiome plays a pivotal role in physiological processes, from nutrient absorption to immune regulation, with significant implications for host health.
On the other, disruptions in this intricate ecosystem, known as dysbiosis, are implicated in age-related diseases, ranging from cardiovascular diseases to neurodegenerative disorders.
Changes in the gut microbiota are characterised by a general decrease in microbial diversity and distinct patterns in the prevalence of certain bacterial genera.

Fecal microbiota transplantation (FMT) studies reveal the causative role of gut dysbiosis in age-related systemic inflammation and decline in adaptive immunity.
Multiple studies have demonstrated the potential to manipulate gut microbiota to rejuvenate the ageing immune system and brain.
Notably, certain probiotics and metabolites have shown promise in restoring a youthful microbiome, thereby enhancing health span and potentially extending lifespan.
These scientific insights into the gut microbiome and ageing pave the way for targeted interventions, potentially involving pre-, pro-, and post-biotics, to improve health in older adults.
Given the heterogeneity of the gut microbiome influenced by factors like diet, genetics, and environment, it’s crucial to employ personalised approaches for maximum effectiveness.
12: Altered intercellular communication
In ageing, there’s a significant shift in the way cells communicate with each other, leading to a sort of “static noise” in the biological system that hinders its optimal functioning.
Normally, our body has hormonal, neural, and neuroendocrine systems that help maintain stability—think insulin for regulating blood sugar or adrenalin for quick energy.
These communication systems begin to malfunction as we age. It’s not just a matter of decreased sex hormones, commonly linked to reproductive ageing, but the disruptions extend to various systems like insulin/IGF1 pathways and even neurotransmitters such as dopamine.
The sources of these communication glitches are often cell-intrinsic, meaning they originate within the cells themselves. These contribute to bigger, system-wide problems like chronic inflammation and weaker immunity against pathogens and irregular cell growth.
Impaired intercellular communication also mess up the healthy dialogue between our human genome and our microbiome, leading to gut imbalances or “dysbiosis”.

Altered cellular communication includes both long-range and short-range communication systems.
For example, the brain has a significant role in how ageing manifests in peripheral organs.
Manipulating specific genes in the brain can actually enhance the longevity of mice, although the exact mechanisms behind these far-reaching effects are not fully understood.
Short-range communication isn’t less intricate; it involves a multitude of molecules released from tissues like white and brown fat, the heart, liver, and muscles.
These secreted factors interact with other cells through various ligands and receptors or even direct cell-to-cell connections, and these interactions can also change with ageing.
Our extracellular matrix (ECM), the ‘scaffolding’ for our tissues also loses its function overtime. Ageing significantly alters its structural components, leading to tissue fibrosis or ‘fibroaging’.
The ECM not only becomes stiffer with age but also affects the function of senescent cells that, in a vicious cycle, further damage to the ECM. This stiffness can trigger other pro-fibrotic pathways, adding another layer of complexity to the ageing process.
Inhibiting certain factors has shown to rejuvenate certain cells in the brains of older mice. Collagen, the protein that provides structure to much of your body, also plays a crucial role.
Learn more about the role of collagen supplements here.
Avea solution: The hallmarks of ageing tree
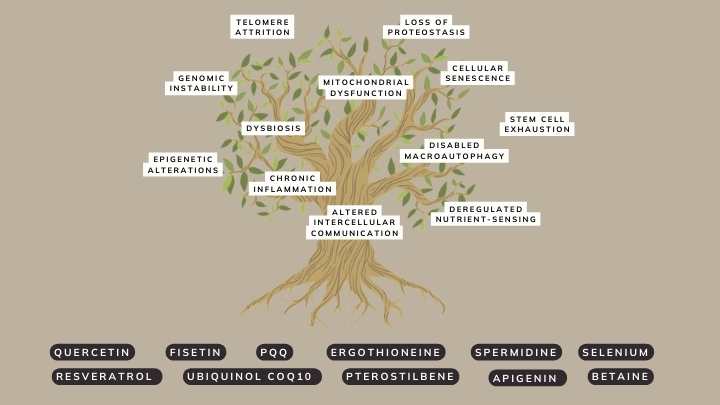
The Avea Reset Bundle is ingeniously designed to target multiple hallmarks of ageing at the same time.
It combines an array of scientifically-backed ingredients that synergistically work to mitigate cellular senescence, boost autophagy, and provide cellular protection against oxidative stress.
- Renew and revitalise your cells with this powerful duo.
- Cell Primer: Clear the path by jump-starting cellular autophagy and the clearing of zombie cells.
- Booster: Nourish your cells, amplifying energy production and cellular resilience.
References