When we hear the word longevity, we often associate it with extreme age – the goal of living past 100, or even 120. But the most meaningful progress in longevity science isn’t about extending lifespan alone. It’s about extending the number of years we remain physically capable, metabolically resilient, and free from chronic disease.
This concept is known as healthspan – the portion of life during which we function independently, maintain physiological balance, and retain a high quality of life.
In other words, it’s not just about adding years to life, but about adding life to those years.
Modern healthcare has added decades to the average human lifespan, but those added years often come with increased burden of illness – cardiovascular disease, neurodegeneration, insulin resistance, or chronic inflammation. The goal of longevity research is to understand, and ultimately influence, the underlying biological processes which drive ageing – so we can slow their progression, delay disease onset, and preserve wellbeing for longer.
In this article, we’ll explore the core hallmarks of ageing, the biological systems that influence how we age, and the interventions – lifestyle, nutritional, and therapeutic – which show the greatest promise for extending healthspan.
In this article
The biology of ageing, and what’s actually happening inside the body
Ageing was once thought to be a passive, inevitable process – the gradual wear and tear of tissues over time. But in recent decades, science has revealed that ageing is not random. It follows a coordinated series of biological shifts which begin at the cellular level and ripple out to affect every major system in the body.
In 2013, researchers published a landmark paper outlining nine core hallmarks of ageing – interconnected mechanisms which drive the biological ageing process. More recently, in 2023, scientists expanded this framework to include three additional hallmarks, forming a more complete picture of how and why we age.
These Twelve Hallmarks of Ageing are now recognised as the foundation for understanding age-related decline, and, more importantly, as levers we may be able to influence through lifestyle, nutritional, and therapeutic interventions.
They are grouped into three categories based on their role in ageing: primary hallmarks (causes of damage), antagonistic hallmarks (responses to damage), and integrative hallmarks (final consequences that lead to functional decline).
The Twelve Hallmarks of Ageing
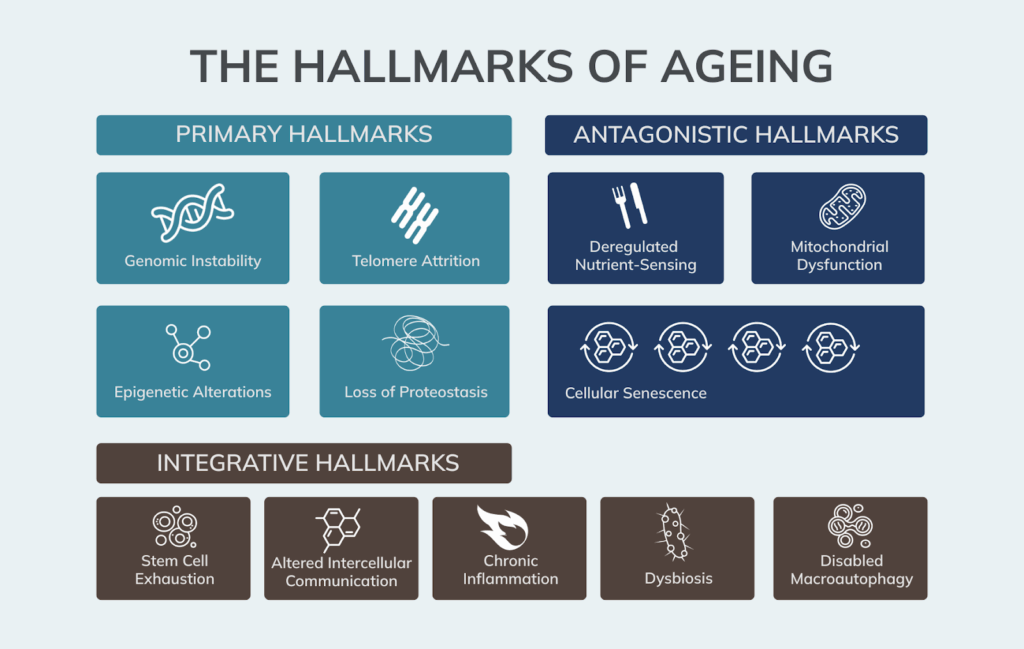
Primary Hallmarks
These represent the root causes of cellular damage that accumulate over time. They are the starting point of biological ageing and include damage to DNA, changes in gene regulation, and breakdowns in protein maintenance.
- Genomic Instability
DNA damage can arise from both internal factors (like oxidative stress) and external exposures (such as UV radiation or environmental toxins). As this damage accumulates, it disrupts cellular function and increases the likelihood of age-related diseases, including cancer. - Telomere Attrition
Telomeres are repetitive DNA sequences that protect the ends of chromosomes. Each time a cell divides, these telomeres shorten. When they reach a critical length, the cell can no longer replicate effectively, contributing to tissue ageing and reduced regenerative capacity. We’ve written more about telomeres in our article here. - Epigenetic Alterations
Epigenetic mechanisms regulate gene expression without changing the underlying DNA sequence. Ageing is associated with widespread epigenetic drift, leading to the inappropriate activation or silencing of genes and the loss of cellular identity. - Loss of Proteostasis
Proteostasis refers to the maintenance of healthy protein folding, function, and clearance. With age, the efficiency of systems like chaperones, proteasomes, and autophagy declines, leading to protein misfolding and aggregation – a key feature in many neurodegenerative diseases.

Antagonistic Hallmarks
These are initially adaptive responses to damage that, when chronically activated or poorly regulated, begin to drive ageing themselves. They include shifts in metabolism, energy production, and cellular stress signaling.
- Deregulated Nutrient Sensing
Pathways like insulin/IGF-1 signaling, mTOR, AMPK, and sirtuins help the body respond to energy and nutrient availability. With age, these pathways become imbalanced, leading to metabolic dysfunction, inflammation, and reduced cellular resilience. - Mitochondrial Dysfunction
Mitochondria produce the energy that cells need to function. Over time, they become less efficient and generate more reactive oxygen species (ROS), which damage cellular components and promote inflammation. - Cellular Senescence
Cells experiencing irreparable damage may enter a senescent state – no longer dividing but remaining metabolically active. Senescent cells secrete pro-inflammatory factors (the SASP, or senescence-associated secretory phenotype) that impair tissue structure and function.

Integrative Hallmarks
These are the downstream effects of both the primary and antagonistic hallmarks. They represent the culmination of cellular ageing and are directly responsible for the loss of tissue function and resilience.
- Stem Cell Exhaustion
Stem cells are responsible for tissue repair and regeneration. Age-related decline in stem cell number and function reduces the body’s ability to maintain and repair tissues, contributing to frailty and slower recovery. - Altered Intercellular Communication
Ageing tissues exhibit changes in how cells communicate – often driven by chronic, low-grade inflammation (“inflammaging”). This disrupts systemic homeostasis and promotes the progression of age-related diseases.
Newly Proposed Hallmarks of Ageing
In 2023, researchers proposed three additional hallmarks based on new evidence that expands our understanding of what drives the ageing process. These additions reflect growing recognition of the roles that autophagy dysfunction, chronic inflammation, and the gut microbiome play in maintaining long-term health and resilience. While more research is still ongoing, these newer hallmarks have become increasingly accepted by the scientific community as central to the ageing process.
- Disabled Macroautophagy
Macroautophagy is the process by which cells break down and recycle damaged components. As we age, this system becomes less efficient, leading to the accumulation of dysfunctional organelles and proteins, contributing to cellular stress and ageing. - Chronic Inflammation
Known as “inflammaging,” chronic low-grade inflammation becomes more common with age and drives tissue damage, metabolic dysfunction, and multiple age-related diseases, even when no infection is present. - Dysbiosis (Imbalanced Microbiome)
The gut microbiome plays a key role in immune regulation, metabolism, and nutrient synthesis. With age, microbial diversity often declines and harmful bacteria can dominate, contributing to systemic inflammation and reduced resilience.
For a deeper dive into the original hallmarks, see our detailed guide on the Hallmarks of Ageing here.
The discovery of these hallmarks has shifted the field of longevity science from managing symptoms to identifying and targeting the root causes of ageing. These mechanisms are not simply markers of age, they are modifiable levers.
Through strategic interventions ranging from lifestyle changes and dietary optimization to advanced therapeutics and nutraceuticals it may be possible to slow, or even partially reverse, some of these biological processes. Understanding the hallmarks provides a scientific foundation for longevity-focused health strategies aimed at extending healthspan, not just lifespan.
In other words: ageing isn’t just something that happens to us. It’s something we can start to understand, and influence.
Longevity pathways: how the body regulates the ageing process
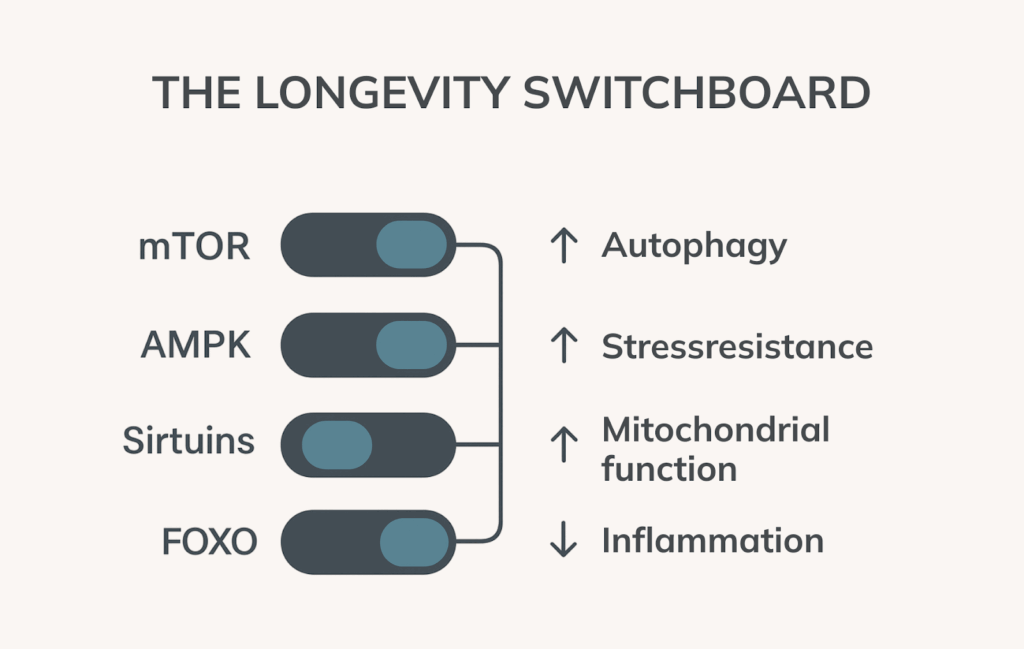
While the hallmarks of ageing explain what goes wrong in the body over time, longevity pathways describe how the body naturally resists or repairs that damage. These pathways are evolutionarily conserved biological circuits that regulate how cells respond to stress, manage energy, and carry out maintenance and repair.
They help the body adapt to challenges like nutrient scarcity or oxidative stress, and are now key targets in longevity research, due to their role in promoting resilience, repair, and metabolic efficiency.
The most studied longevity pathways include:
mTOR (mechanistic Target of Rapamycin)
mTOR is a key growth regulator that responds to nutrient availability, particularly amino acids and insulin. When activated, mTOR stimulates protein synthesis, cell growth, and anabolic metabolism. While essential for development and recovery, chronic mTOR activation (e.g. from constant food intake and low physical activity) is linked to accelerated ageing and diseases like cancer.
Inhibiting mTOR activity periodically through caloric restriction, fasting, or compounds like rapamycin has been shown to extend lifespan in many species. This is largely due to enhanced autophagy, a cellular recycling process that clears damaged proteins and organelles, supporting long-term cell health.
AMPK (AMP-activated protein kinase)
AMPK functions as a cellular fuel gauge, activated when energy is low (e.g. during exercise, fasting, or caloric restriction). Once activated, AMPK triggers processes that boost energy production and reduce energy consumption. It promotes fat oxidation, glucose uptake, mitochondrial biogenesis, and autophagy, shifting the body from a growth state to a repair state.
AMPK is considered one of the most promising targets for improving metabolic health and resilience against age-related decline.
Sirtuins (SIRT1–7)
Sirtuins are a family of NAD⁺-dependent enzymes that regulate cellular stress responses, DNA repair, mitochondrial function, and inflammation. They are central to the health benefits of caloric restriction and are increasingly studied in the context of ageing and age-related disease.
As NAD⁺ levels decline with age, sirtuin activity drops, contributing to reduced cellular resilience. Boosting NAD⁺ through precursors like NMN or NR, or activating sirtuins via compounds like resveratrol, may support healthier ageing by enhancing mitochondrial efficiency and genomic stability. We’ve written more on the science behind NAD+ in our article here.
FOXO Transcription Factors
FOXO proteins (Forkhead box O) are transcription factors that regulate genes involved in longevity, stress resistance, DNA repair, and antioxidant defense. They are activated in response to oxidative stress, growth factor withdrawal, and caloric restriction.
In model organisms, increased FOXO activity is associated with longer lifespan and protection against age-related diseases. FOXO proteins mediate many of the beneficial effects of interventions like fasting, exercise, and AMPK activation by encouraging cells to prioritize maintenance over growth.
Together, these longevity pathways serve as internal control systems guiding how cells maintain balance, repair damage, and adapt to stress. By supporting or activating these systems through nutrition, fasting, exercise, or targeted compounds, we may be able to influence how we age from the inside out.
Interventions that support longevity: what we know so far

While we can’t stop the clock, emerging research suggests we can slow its pace by supporting the biological systems that regulate ageing. From calorie restriction and exercise to targeted nutrients and compounds, multiple interventions have been shown to influence the hallmarks and pathways of ageing in meaningful ways.
Here’s a look at some of the most promising strategies:
Nutritional Interventions
Caloric Restriction (CR)
Reducing overall calorie intake without causing malnutrition has consistently extended lifespan in laboratory animals, from yeast to primates. CR lowers oxidative stress, enhances autophagy, and suppresses overactive pathways like mTOR while activating protective ones like sirtuins and AMPK.
Intermittent Fasting / Time-Restricted Eating
Time-restricted eating mimics many of the effects of CR without reducing overall calories. It promotes metabolic switching, improves insulin sensitivity, stimulates autophagy, and activates energy-sensing pathways (AMPK and sirtuins). Fasting can also enhance mitochondrial function and may reduce inflammation systemically.
Protein Quality & Quantity
Certain amino acids – like methionine and BCAAs – may overstimulate mTOR when consumed in excess. Reducing these in midlife, or shifting to more plant-based protein sources, may moderate growth signals and support healthy ageing. However, protein requirements may increase again in later life to preserve muscle mass.
Physical Activity
Endurance and Resistance Exercise
Both forms of exercise have profound anti-ageing effects. Endurance training boosts mitochondrial density and cardiovascular resilience. Resistance training preserves muscle mass, supports insulin sensitivity, and increases circulating NAD⁺. Both types activate AMPK, promote autophagy, and enhance cognitive health through increased brain-derived neurotrophic factor (BDNF).
Supplementation & Nutraceuticals
We’ve written more in depth on supplementation in our article here.
NAD+ Precursors (NMN, NR)
NAD+ fuels sirtuin activity and mitochondrial repair but declines with age. Supplementation with precursors like NMN and NR can increase intracellular NAD⁺ levels, potentially enhancing cellular energy metabolism, DNA repair, and stress response pathways. Clinical trials show promise in improving muscle function and cardiometabolic markers. We’ve written more on the science behind NAD+ in our article here.
Polyphenols (Resveratrol, Pterostilbene, Fisetin, Quercetin)
These plant compounds activate key longevity pathways (sirtuins, AMPK, FOXO) and may mimic calorie restriction effects. Fisetin and quercetin also act as senolytics – clearing senescent cells and reducing chronic inflammation. Their antioxidant and anti-inflammatory properties make them candidates for extending healthspan. We’ve written more on these topics in our articles here:
- Resveratrol supplements for longevity: benefits, dosages, side effects
- Pterostilbene supplement: benefits, dosage, and side effects
- Quercetin v.s. Fisetin: which one is better?
Magnesium Bisglycinate & Adaptogens
Magnesium plays a role in hundreds of biochemical reactions, including those governing nervous system health and circadian rhythm. Magnesium bisglycinate is especially well-tolerated and bioavailable. Adaptogens like ashwagandha and holy basil help the body manage stress by modulating cortisol and protecting mitochondria from stress-induced damage. We’ve written more on Magnesium in our articles here.
Omega-3s (EPA/DHA)
These essential fatty acids are anti-inflammatory and neuroprotective. They support heart health, stabilize cell membranes, and may preserve telomere length. Omega-3s are also being studied for their ability to modulate gene expression and reduce age-related cognitive decline. We’ve written more on Omega-3s in our article here.

Emerging Therapies
Senolytics
These compounds selectively eliminate senescent cells, which contribute to chronic inflammation and tissue breakdown. In animal models, senolytics have improved mobility, organ function, and resilience. Fisetin and quercetin are among the most studied senolytic agents. We’ve written more on Senolytics in our article here.
Rapamycin & mTOR Inhibitors
By selectively suppressing mTOR, rapamycin can trigger autophagy and cellular repair mechanisms. In nearly every species studied, it has extended lifespan, even when administered later in life. Low-dose or intermittent dosing in humans is under investigation, with early trials showing promise for immune rejuvenation and metabolic benefits.
As longevity science continues to evolve, the most promising strategies involve a layered, systems-based approach – targeting multiple hallmarks and pathways in parallel through sustainable, evidence-based interventions.
Building a Longevity Routine
Scientific progress means little without practical application. While the biology of ageing is complex, many of the most impactful interventions are surprisingly simple. The key lies in consistency and systems-thinking – addressing cellular health, metabolic flexibility, and physiological resilience as interconnected parts of a long-term strategy.
Here’s how to start integrating longevity principles into everyday life:
Prioritise sleep
Sleep isn’t just rest – it’s a critical window for cellular repair, memory consolidation, and metabolic regulation.
Poor or inconsistent sleep has been linked to mitochondrial dysfunction, increased inflammation, and accelerated biological ageing:
- Aim for 7–9 hours of high-quality sleep per night
- Support circadian rhythms by getting natural light exposure in the morning and avoiding bright screens at night
- Supplement with compounds like magnesium bisglycinate, saffron extract, or L-theanine, which promote deeper, more continuous sleep without sedation
We’ve written more on the science of sleep in our articles below:
- Why you’re still waking up tired-even after 8 hours of sleep
- Why you’re sleeping worse during menopause, and how to fix it
Regular physical activity
Regular physical activity activates nearly every longevity pathway, from mitochondrial biogenesis to autophagy. Both aerobic and resistance training contribute uniquely to healthspan. We’ve written more on the benefits of physical activity on longevity here.
- Cardiovascular training (like walking, swimming, or cycling) supports heart health, metabolic flexibility, and brain function
- Strength training helps preserve lean muscle mass, maintain insulin sensitivity, and prevent frailty
- Even short bouts of movement throughout the day can reduce inflammation and counteract the effects of prolonged sitting
Eat with intention
What and when you eat influences everything from inflammation to cellular regeneration.
- Focus on nutrient-dense foods – colorful vegetables, legumes, omega-3-rich fish, and healthy fats
- Prioritize plant diversity and fiber to support a healthy gut microbiome, now considered a key player in longevity
- Try intermittent fasting or time-restricted eating to activate repair processes like autophagy and improve metabolic health. We’ve written more on intermittent fasting in our articles below:
Be mindful of protein quality and quantity, especially with age. Overactivation of mTOR via excess protein may accelerate ageing; balance is key
Manage stress
Chronic psychological stress is one of the most potent accelerators of ageing, contributing to telomere shortening, hormonal imbalance, and systemic inflammation.
- Practice daily stress-reducing rituals like deep breathing, meditation, or journaling
- Use adaptogens (e.g., ashwagandha, rhodiola, or holy basil) to support the hypothalamic-pituitary-adrenal (HPA) axis
- Prioritize rest and recovery, not just productivity. Longevity is a marathon, not a sprint
Support with Targeted Supplementation
When used strategically, high-quality supplements can support biological systems involved in ageing and enhance the benefits of your daily habits.
- NAD+ boosters (like NMN or NR) to support mitochondrial health and energy metabolism
- Magnesium bisglycinate for nervous system balance and sleep
- Omega-3s (EPA/DHA) for inflammation control and cellular membrane integrity
- Senolytics (e.g. quercetin, fisetin) to target and clear senescent cells
Always consult with a healthcare provider before starting a new supplement protocol.
A longevity routine isn’t about doing everything at once. It’s about thoughtful layering: small, evidence-based habits that compound over time.
Is biological age more important than chronological age?
Two people can be the same age in years, but decades apart biologically.
Chronological age is the number of years you’ve been alive. Biological age, on the other hand, reflects the functional status of your cells, tissues, and organs, and is shaped by lifestyle, environment, and molecular damage.
Recent advances in epigenetic testing now allow us to measure biological age through markers like DNA methylation. These tests don’t just estimate how “old” your body is acting – they also show how quickly you’re ageing and whether your current routine is slowing or accelerating that process.
Understanding your biological age gives you a clearer picture of your long-term health trajectory. More importantly, it empowers you to take action. By addressing the root mechanisms of ageing – mitochondrial decline, cellular senescence, chronic inflammation – you can improve healthspan even if your lifespan is still unknown.
A longevity mindset: the power of prevention
Perhaps the most profound shift in longevity science is a philosophical one:
- From treating disease to preserving function
- From reacting to symptoms to addressing root causes
- From isolated fixes to integrated routines
This mindset values:
- Proactivity over passivity: Supporting health before dysfunction begins
- Systems-thinking: Viewing the body as an interconnected, dynamic system
- Compounding habits: Small, science-backed actions that add up over time
Just as compound interest grows wealth, compound habits grow health.
Longevity isn’t just about adding years to life, but about adding life to those years. Energy, clarity, strength, presence. And it starts with the choices you make today.
At AVEA: Science-Backed Support for a Longer, Healthier Life

At AVEA, our mission is to empower people to build effective longevity routines through credible research and high-quality formulations. Our product development is guided by longevity researchers and clinicians from institutions like ETH Zürich, and our supplements are designed to target the core pillars of healthspan: cellular health, cognitive clarity, metabolic function, skin vitality, and sleep quality.
When your cells function better, everything else does too.
Let’s help you stay healthier, longer.